The Periodic Schemata of the Elements
By Charles William Johnson
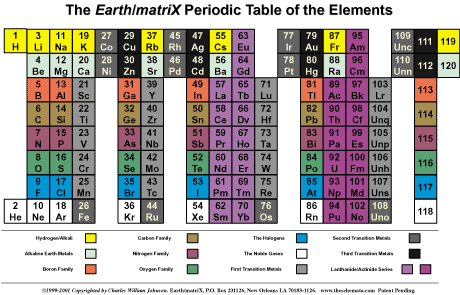
The Mendeleev-based periodic table of the
elements currently in use, represents the result of much thought
and research about the elements and their atomic nature. The table
of the elements reflects the atomic number of the elements and
their periodicity. The periodicity, which reflects the manner
in which the shells and sub-shells of the atoms are filled by
electrons, represents only one particular aspect about the elements.
The conventional periodic
table reflects what is called the aufbau design, which
represents a progression of numbers; in this case, that of the
atomic number of the elements. The table, however, contradicts
the aufbau concept in reality, because there are large
gaps within among the primary (representative) elements, as well
as in relation to the tertiary elements (transition and inner
transition elements). The latter case, the Lanthanoids and the
Actinoids, lie completely outside of the main body of the periodic
table, thereby effectively breaking down the aufbau design.
Because of the fragmented
placement of the elements within the conventional periodic table,
it is impossible to visualize certain patterns concerning data
related to the elements and their atoms. In fact, the Mendeleev-based
table can only convey a half dozen or so visualizations of the
charteristics of the elements, such as covalent radii, temperature,
shells, valences, in addition to a few others.
Furthermore, the fact that
the atoms/elements are presented in a block-like formation, does
not allow the concept of periodicity to be expressed upon the
conventional table, other than in terms of the abstracted numbers.
There is no spacing between the periodicity of a proportional
nature that might convey the idea of the periods.
Our studies in ancient
artwork have led to re-conceptualize the layout of the periodic
table. At first, our intention was simply to bring the Lanthanide
and Actinide series into the main body of the table. We based
our studies upon the idea of conceiving the electron configuration
of the atoms/elements from an ideal position. We constructed an
electron count that would eliminate, or re-accommodate the so-called
irregularities in the electron configuration of the atoms.
Once we re-structured the
arrangement of the atoms/elements according to this ideal count,
we then realized that a totally distinctive schematic design appeared.
We have called this design the schema. The reason
for assigning the re-structuring a distinct name is that the schema
accommodates much more information than the Mendeleev-based table.
The schema accommodates the concept of periodicity, as
well as, many other aspects of the elements, which are unavailable
for viewing on the current periodic table.
In other words, had we
continued to call it simply "the" or "a" periodic
table, we would not be conveying the idea of the additional data
that may be registered on the schema. Therefore, in the complete
study, we present different schemata, which treat many other aspects
of the elements and their atoms; not just that of periodicity.
The schema design, then,
allows us to visualize many different patterns regarding the behavior
and characteristics of the atoms/elements. Furthermore, the schema
may be scrolled, vertically or horizontally, and in this way an
infinite number of patterns make their appearance. The schema,
then, accommodates data regarding well-known patterns, as well
as, revealing new, original patterns, which have not been observed
or detected until now.
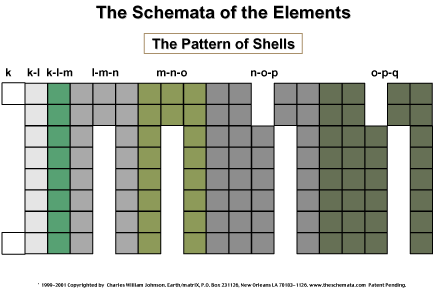
From the previous illustration,
it is now possible to view the progressive numerical ordering
of the elements on a compact design, whereby the gaps now lie
only within the tertiary pattern. The primary, or representative
elements, are no longer separated as in the Mendeleev-based table.
Furthermore, it should be obvious that the periodicity may now
be represented in a spatially proportional manner, unlike the
current table.
Beyond that, the schema
offers the placement of much more data upon its design and thereby
allows the visualization of an infinite array of patterns reflected
by the elements and their atoms. Let us note some of the features
that may be accommodated on the schema and the current periodic
table, in order to realize that the schema is much broader than
the mere concept of a periodic table. Periodicity, as we stated,
is simply one characteristic of the elements/atoms; the schema
allows for the placement and pattern recognition of many different
characteristics. We shall note which characteristic may be exemplified
on either table.
| Characteristic
Pattern: |
Schemata
of the Elements |
Mendeleev
Table |
| Atomic Number (aufbau
Sequential) |
Yes |
No |
| Atomic Weight |
Yes |
Yes |
| Cycles (compact) |
Yes |
No |
| Densities |
Yes |
No |
| Electro-negativity |
Yes |
No |
| Families |
Yes |
Yes |
| Families (without
gaps) |
Yes |
No |
| Groups |
Yes |
Yes |
| Ionization |
Yes |
No |
| Metals, Metalloids,
Non-Metals |
Yes |
Yes |
| Molecular formulae |
Yes |
No |
| Odd/Even (Stable
Isotopes) |
Yes |
No |
| Periodicity (numerical) |
Yes |
Yes |
| Periodicity (spacing) |
Yes |
No |
| Projections: |
Yes |
No |
| 168-Elements |
Yes |
No |
| 216-Elements |
Yes |
No |
| Proton, Neutron,
Electron Counts |
Yes |
No |
| Radioactivity (compact) |
Yes |
No |
| Sectors |
Yes |
No |
| Shells |
Yes |
Yes |
| Symmetry of First
Twenty Elements |
Yes |
No |
| Valence electrons |
Yes |
Yes |
| Weighable/unweighable
elements |
Yes |
No |
All of the above characteristics,
among other additional ones analyzed in our manuscript, refer
to the possibility of producing visual patterns upon the Mendeleev-based
periodic table or upon the schemata of the elements. For example,
the metals, metalloids and non-metals are shown on almost every
current periodic table. Yet, these are not illustrated according
to a spatial proportion determined by their periodicity. Each
one of the patterns detected and illustrated upon the schema,
mentioned above, in fact also reflect the periodicity of each
of these visualizations, as well as their underlying sectors and
cycles.
None of these cited patterns
are available on the conventional, Mendeleev-based periodic table.
The above chart indicates that any one of those features, whose
data are registered on the current periodic table, shall not produce
a recognizable pattern. Whereas the schema immediately produces
a pattern rendered as of the data. And, we should note that patterns
ultimately permit the concept of prediction and extrapolation
of the data. For this reason the schema allows for projections
of the patterns in order to recognize the elements that follow
on the table up to 166 (168) or 216 (218) elements, depending
upon the cycle under consideration.
As of the Mendeleev-based
table, projections for a 168 and 218 periodic table exist. However,
the schema shall indicate that the following cycles terminate
on 166 elements and 216 elements. Further, we present schemata
which illustrate the non-concordance of current projections.
©1999-2013
Copyrighted by Charles William Johnson. All rights reserved. Patent
Pending
|

