
|
|
The Conventional Periodic Table of the Elements:
A Certain Disorder
By Charles William Johnson
A Certain Disorder in Presentation
One might expect to find a single format for the layout of the conventional periodic table. However, there are numerous, almost contradictory forms of. Presentation. We shall offer the main ones, with a reference bibliography. We are not concerned with critiquing a particular layout, but rather noting the the variety of conventional tables now in existence.
The random placement of the Actinide Series (57-71) and the Lanthanide Series (89-103) on the conventional periodic table of the elements, makes it quite impossible to produce color-coded visual patterns on these tables. This feature questions the scientific nature of the conventional periodic table, given the fact that its format is not a constant, but changes accordingly with a particular author.
A Conventional Periodic Table of the Elements
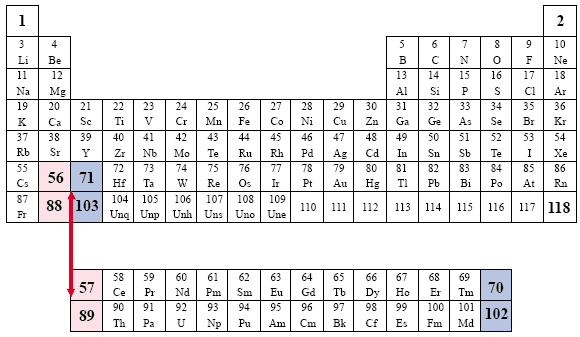
The progressive numeric, sequential order is interrupted.
In Search of a Format
There are many periodic tables with distinct formats. We shall present the most commonly cited tables within the scholarly literature in chemistry.
The vertical columns on the conventional periodic table indicate the groups, now numbered one through eighteen by the IUPAC.
However, the Actinides series and the Lanthanide series are said to be within the third group. Yet, their placement is random, and there is no criterion for placement on the conventional table. This randomness in the format denies any possibility of utilizing the conventional periodic table as a generator of color-coded visual patterns.
There is no single format or presentation of the conventional periodic table today. There are multiple presentations, with many different arrangements of the elements concerning the Actinide series and the Lanthanide series.
One the main reasons for this is the fact that the elements numbers 71 and 103 are yet to be permanently classified. The reason for this, concerns the fact that these two elements share characteristics of a dual nature and may be classified in one or other of the groups, depending upon the feature being analyzed.
An Early Example of the Periodic Table Based on the Mendeleev Form
|
The Groups are represented in the rows and the Periods are represented in the columns of numbers, which correspond to the atomic numbers of the elements. Note the manner in which the elements are dismembered, and the aufbau, or progressive numbering system, is broken. The Lanthanides are represented by an asterisk (*, elements 58 through 71) and the Actinides are represented by two asterisks (**, elements 90 through 103) |
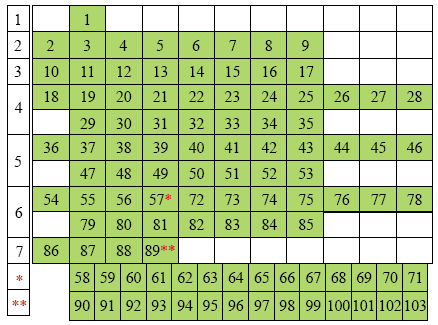 |
The Actinide Series and the Lanthanide Series have enjoyed different nomenclature over the years:
| The Actinoids | The Actinoid Series | The Actinides | The Actinide Series |
The Lanthanoids [Lanthanoid is not in the dictionary.]
| The Lanthanoid Series | The Lanthanides |
Actinoid:
adjective, "resembling a ray; exhibiting radial symmetry"
Actinide Series: A series of heavy radioactive
metallic elements beginning with Actinium (89)
or Thorium (90) and ending with atomic element
number 103.
Lanthanide, noun, a binary compound of Lanthanum
(57).
Lanthanide series: the group of rare-earth
metals, often including Lanthanum and sometimes Yttrium.
Strictly speaking, then, the concept of "actinoid" should not be employed as a noun, and lanthanoid simply has never been accepted as a proper word. Yet, this has stopped these words, and other strange combinations thereof from being employed in the scientific vernacular.
The dismembered format denies any possibility of presenting many of the color-coded, visual patterns that may presented on The Schemata of the Elements.
Numerous, inconsistent ways for the placement of the Actinide and the Lanthanide Series are situated on the conventional periodic table. There is no single format that is followed in the scientific literature on chemistry and physics regarding these series.
Besides the confusion regarding elements 71, 90, and 103, the two series lie randomly below the main body of the periodic table, in extreme positions of placement. This fact alone should suffice to establish the impossibility of displaying thereupon, specific color-coded, visual patterns.
The conventional table also interrupts the sequential numbering of atomic numbers of the elements, which is another reason that some patterns are broken up, and impossible to discern.
Popular Periodic Table
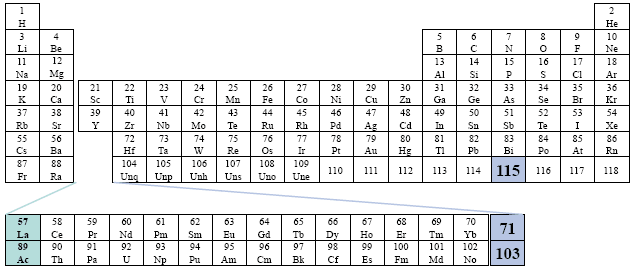
This table considers elements 57 - 71 to be within the Actinide Series, and elements 89 through 103 to be within the Lanthanide Series. All of the elements are placed visually, outside of the main body of the table. Far left placement.
A Conventional Periodic Table of the Elements
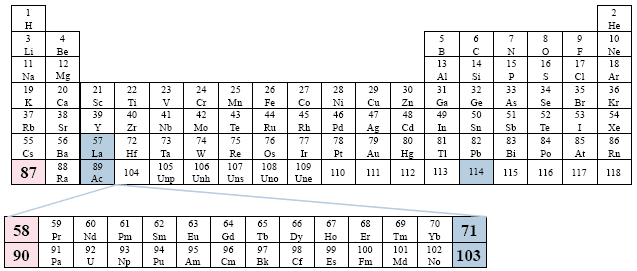
Example: World Book, Inc.
This table also considers the same relationship as the previous table, but now places only the elements 58-71, and 90-103 outside of the table. For the sake of symmetry, the two series begin as o the first column/group. Far left placement.
A Popular Periodic Table
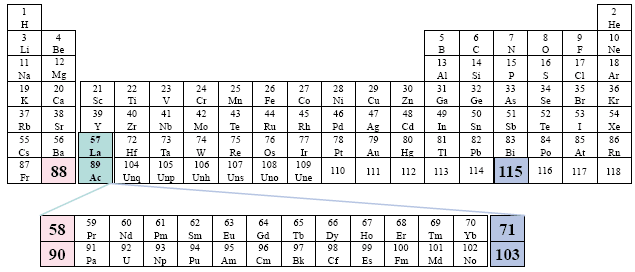
Examples: Choppin
This table repeats the previous pattern, only now the two series begin along the second group. There is no relationship in this sense to the specific group under which the two series are placed. However, we view this random-like placement as a certain disorder in presentation.
A Conventional Periodic Table of the Elements
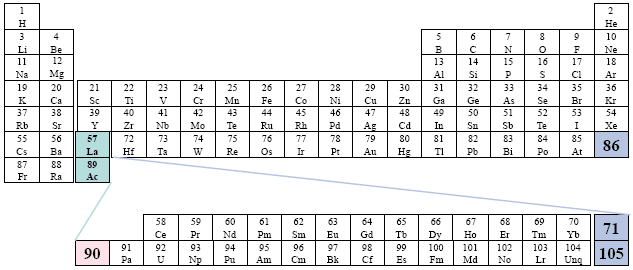
Example: Time-Life Books
This presentation of the two series is somewhat more confusing than normal.
A Conventional Periodic Table of the Elements
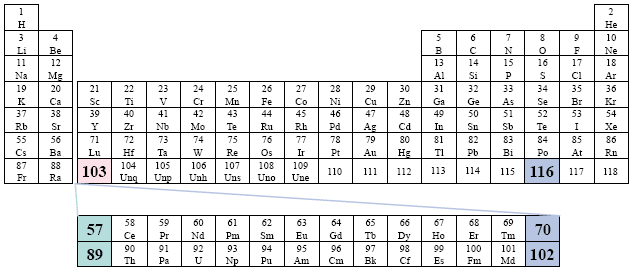
Examples: Metcalfe; Olmsted.
This version of the conventional periodic table presents the Actinide Series as 57 - 70, and the Lanthanide Series as elements 89 - 103.
A Conventional Periodic Table of the Elements
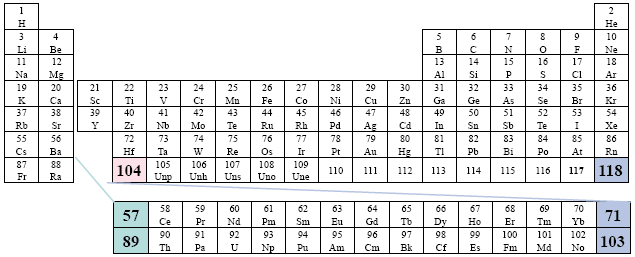
Example: NIST (National Institute of Standards and Technology)
This version is the more generally accepted placement, however, there is a obvious gap that attempts to suggest how the two series fit within the third column (group). Far right placement.
A Conventional Periodic Table of the Elements
Examples: Smoot; Wilbraham.
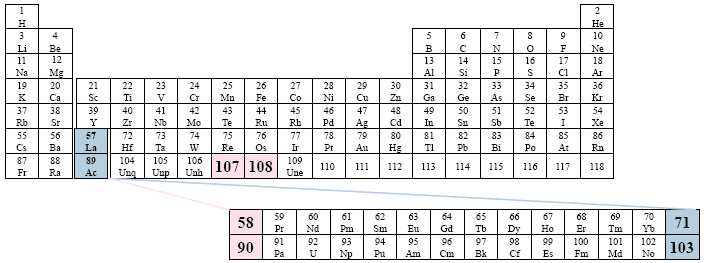
We have viewed this particular option earlier, but now the two series have been moved to the right one column, thus beginning under element 104.
A Conventional Periodic Table of the Elements
Examples: ACS (American Chemical Society); Feigl; Heiserman.
A Conventional Periodic Table of the Elements
Example: Cotton.
A redundant visual presentation for emphasis.
A Conventional Periodic Table of the Elements
Examples: Los Alamos Laboratory; Handbook of Chemistry and Physics
A Conventional Periodic Table of the Elements
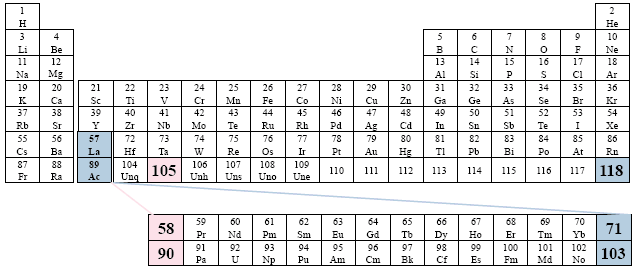
Examples: Bailar; Brown; Chang; Herron; Masterton; Sackheim.
A Conventional Periodic Table of the Elements
Example: Ucko
This presentation represents an extreme placement to the right.
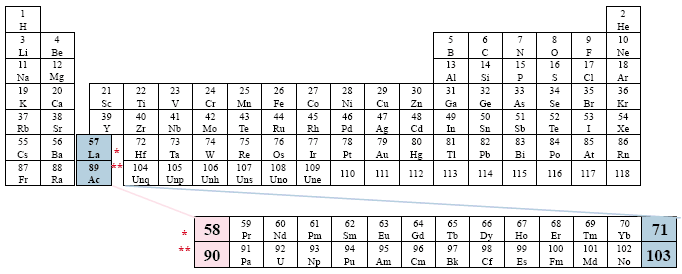
A Conventional Periodic Table of the Elements
Examples: Brady
This version presents the two series in between the columns (groups) of the main body of the table, in an apparent effort to emphasize the non-existent relationship among the different groups and the two series. The placement of the two series is the farthest right that we have found in the literature.
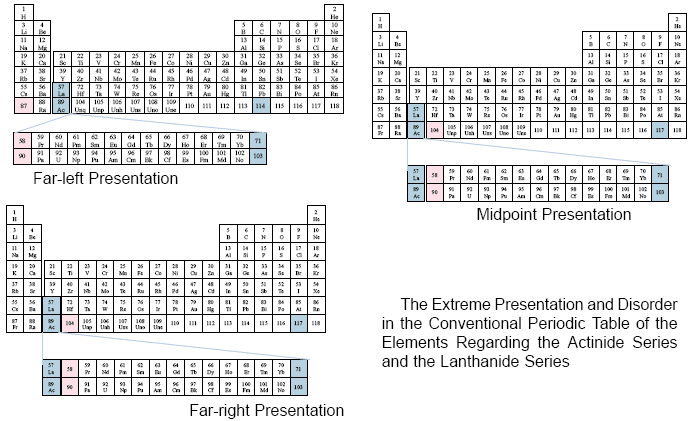
From the previous examples, translated from the different sources cited for each table, one may observe the disorderly manner in which the Actinide Series and the Lanthanide Series of elements are placed upon the conventional periodic table.
Each table shows how these two series fit within the third group of elements. However, there is no relationship to the vertical columns of groups, other than that specific relationship. Also, at times some elements (71, 103) are considered to be within the two series, while other authors consider those same elements to lie outside of the two cited series.
Due to the disorderly manner in which the two series are presented, it is impossible to visualize specific relationships among the elements on the main body of the table in relation to these two series.
The Expanded (Long) Periodic Table [Werner, 1905]
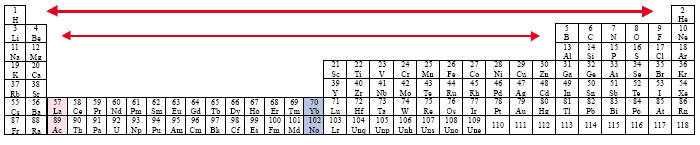
Example: Timmreck
Some authors today utilize a variation of this expanded or long periodic table in an attempt to resolve the placement of the Actinide and Lanthanide Series, by incorporating them into the main body of the table.
The problem that results concerns the tremendous gaps that open up among the Representational Elements, especially for the regular pattern among the first twenty elements.
Bibliography
A Periodic Table of the Elements, Time-Life
Books, 1987. [One-sheet table] American Chemical Society, ACS, Periodic
Table of the Elements, 1998. [One-sheet table]
BAILAR, John C.; MOELLER, Therald; KLEINBERG, Jacob; GUSS, Cyrus O.; CASTELLION,
Mary E.; METZ, Clyde, Chemistry, Academic Press, New York, 1978. [Textbook]
BRADY, James E., General Chemistry: Principles & Structure, John Wiley,
New York, 1990. [Textbook]
BROWN, Theodore L.; LEMAY, H. Eugene, Chemistry, The Central Science,
Prentice-Hall, New Jersey, 1985. [Textbook]
BUELL, Phyllis; GIRARD, James, Chemistry, An Environmental Perspective,
Prentice Hall, New Jersey, 1994. [Textbook]
CHANG, Raymond, Chemistry, Random House, New York, 1984. [Textbook]
Chemistry Today, World Book, Inc., Chicago, 1984. [Popular Encyclopedia]
CHOPPIN, Gregory R.; SUMMERLIN, Lee R., Chemistry, Silver Burdett, New
Jersey, 1978. [Textbook]
COTTON, F. Albert; DARLINGTON, C. Leroy,; LYNCH, Lawrence D., Chemistry:
An Investigative Approach, Houghton Mifflin, Boston, 1076. [Textbook]
CRC Handbook of Chemistry and Physics, 78th Edition, 1997-1998, CRC Press,
Boca Raton, 1997. [Manual]
FEIGL, Dorothy M.; HILL, John W., General, Organic & Biological Chemistry,
Burgess, Minnesota, 1986. [Textbook]
HEISERMAN, David L., Exploring Chemical Elements and Their Compounds,
TAB Books, 1992. [Popular Book]
HERRON, J. Dudley; KUKLA, David A.; DISPEZIO, Michael A.; SCHRADER, Clifford
L.; ERICKSON, Julia Lee, Heath Chemistry, D.C. Heath, Lexington, 1987.
[Textbook]
KOTZ, John C.; PURCELL, Keith F., Chemistry & Chemical Reactivity, Harcourt
Brace Jovanovich, Fort Worth, 1991. [Textbook]
MASTERTON, William L., Chemistry, Holt, Rinehart and Winston, New York,
1980. [Textbook]
MERRILL PUBLISHING Co., Periodic Table, Merrill Publishing Company, 1989.
[One-sheet table]
METCALFE, H. Clark; Williams, John E.; Castka, Joseph E., Modern Chemistry,
Holt, Rinehart and Winston, New York, 1982. [Textbook]
NIST, National Institute of Standards and Rechnology, US Department of
Commerce, Periodic Table, Atomic Properties of the Elements, NIST, Washington,
D.C., March, 1999. [One-sheet Chart]
NASCO, Periodic Table of the Elements, Learning Laboratories, 1996. [One-sheet
table]
OLMSTED III, John; WILLIAMS, Gragory M., Chemistry, The Molecule Science,
Mosby, St. Louis, 1994. [Textbook]
Periodic Table of the Elements: Atomic Properties, BarCharts, Inc., 1999.
[One-sheet table]
Ready Reference, Periodic Table of the Elements, Instructional Fair, Grand
apids, 1995. [One-sheet table]
SACKHEIM, George; LEHMAN, Dennis D., Chemistry for the Health Sciences,
Macmillan Publishing, New York, 1994. [Textbook]
TIMMRECK, Roy, Power of the Periodic Table, Royal Palm, Anchorage, 1991.
[Polemical text]
UCKO, David A., Basics for Chemistry, Academic Press, New York, 1982.
[Textbook]
WILBRAHAM, Anthony C.; STALEY, Dennis D.; MATTA, Michael S., Chemistry,
Addison-Wesley, Menlo Park, 1995. [Textbook]
©2001-2014 Copyrighted by
Charles William Johnson. All rights reserved.
Earth/matriX P.O. Box 231126 New Orleans, Louisiana, 70183-1126
More topics...
- How to Read Schemata
In order to overcome these shortcomings of the conventional periodic table, we are presenting a rearrangement of the elements in a compact schematic design. - Handbooks
on Chemistry and Physics,
The names of the elements are historically accidental ones. - Forget the Periodic
Table?.
The traditional periodic table denies a visualization of the concept of the periodicity, the very concept that is supposedly represents.
- Symmetry
on the Earth/matriX Thermodynamic Temperature Scale:
Absolute Zero | Melting Point | Boiling Point | Critical Point of Selected Elements, Together with Triple Points
PRESS RELEASE |
ESSAYS
|
REVIEWS
EVENTS
Beyond the Periodic Table of the Elements. The Neutronic Periodic Table. Specialized neutronic schemata were presented, revealing new and original patterns of symmetry. IMAGES
|
THE
STORE
LINKS
EXTRACTS
|
||
Physical and Chemical Constants by Earth/matriX
|
|||||